
FASTinov AST kits are CE-marked, approved for sale in the European Union under IVD Directive. They are not FDA-cleared and are not yet available for sale in the U.S. Availability in each country depends on local regulatory clearance.
LEARN MORE
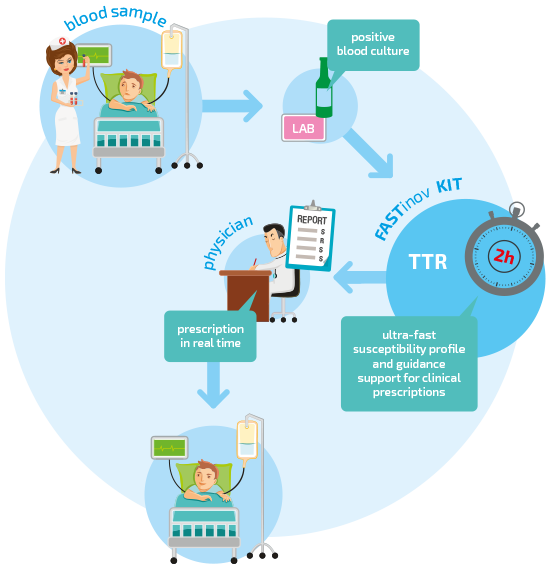
Phenotypic Antimicrobial Susceptibility in 2 hours directly from blood cultures.
FASTinov AST delivers phenotypic Antimicrobial Susceptibility Testing within 2 hours, with superior precision for both gram negative and gram positive panels.
Now CE-Marked
The world’s fastest antibiogram
 LEARN MORE
LEARN MORE
The FASTinov AST uses flow cytometry to detect lesions that happen in the first minutes of contact between bacteria and antibiotics.
Because it doesn’t depend on bacterial growth, the results are always obtained in less than 2 hours.
FASTEST phenotypic susceptibility test for both gram negative and gram positive bacteria

 LEARN MORE
LEARN MORE


 © 2022
© 2022